Silver is a natural antimicrobial which kills bacteria, viruses, fungi/yeasts, molds and protozoa parasites
How To Be Sure It's Real Colloidal Silver
Many companies use the term "colloidal silver" to describe their products. But, very few actually are. Understanding what true colloidal silver is will help you purchase an all-natural product that is effective at persistently killing microbes (bacteria, viruses, fungi/yeasts, molds and protozoa parasites) on surfaces. The following is as brief of an accurate explanation as you're likely to find.
Properties of a colloidal solution
A colloidal solution of silver nanoparticles in very pure water (real colloidal silver)
Color is the simple way to indicate silver nanoparticles are present
The darkness of the color is an indication of the nanoparticle concentration
Products that have added proteins or polymers
Clear products that contain ions with few nanoparticles
Real colloidal silver will persist and continue killing microbes
True colloidal silver must first meet the definition of a colloid:
A mixture in which particles of one substance are held inside another substance.
:-
Particle size of 1-1000nm (nanometer), also known as nanoparticles. Note: a silver ion is 0.3nm and becomes a true solution in water.
-
Colloidal particles pass through micron level filter paper but not through parchment paper. Silver ions are small enough to pass through parchment.
-
Colloidal particles are individually not seen to naked eye but can be observed through a very high powered microscope.
-
Colloidal solutions are translucent with color determined by the light absorbed by the particles.
-
Colloids show the Tyndall effect of scattering light. To see this, in a dark room, shine a laser pointer through the colloidal silver in a clear container. CAUTION: DO NOT SHINE LASER INTO EYES OF ANYONE, INCLUDING PETS.
A colloidal solution of silver nanoparticles
in very pure water (real colloidal silver) must contain
nothing else to accomplish the particle suspension. The nanoparticles
are only suspended by mutual electrical repulsion, also known as
zeta potential. Very pure water and zeta potential
can suspend almost 50ppm (parts per million) of elemental silver in
the form of nanoparticles and ions combined. Anything more must be
assisted by something else, usually proteins or polymers which is not a
colloidal solution or real colloidal silver.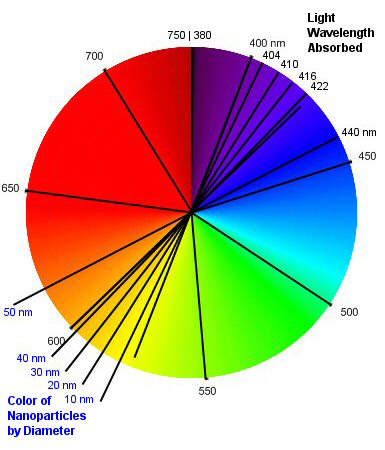
Color is the simple way to indicate silver nanoparticles are present. Those small enough to be suspended in pure water absorb about 400 to under 440nm wavelength light, depending upon their size from 1nm to under 50nm, respectively (based on "Silver Nanoparticles: Properties and Applications" by Steven J. Oldenburg, Ph.D., President - nanoComposix, Inc. posted at Sigma-Aldrich). This results in a color from greenish yellow through yellow to orange on the visual spectrum, again respectively. Generally, the nanoparticle sizes in real colloidal silver vary throughout that range and produce an amber translucent color.
A particle distribution averaging closer to the 50nm size will appear more orange or brown. Conversely, a particle size distribution average closer to 10nm will appear more yellow and a 1nm distribution will appear greenish yellow. Smaller nanoparticles have the largest combined surface area for the same ppm silver concentration, making them more effective.
The darkness of the color is an indication of the nanoparticle concentration, assuming nothing has been added. Darkness is a separate property from color and yellow can be darker than an amber in that respect.
Products that have added proteins or polymers (known as silver proteins) generally have a color like real colloidal silver, which can be quite dark, due to the higher concentration of silver particles suspended by the additives. Proteins and polymers can also suspend even larger and less effective particles, resulting in an orange to reddish color. Very large silver particles begin to have a gray/blackish color which, when mixed with amber or orange, produces a brownish color.
Proteins or polymers coat the silver particles, partially isolating them from their surroundings for less contact and effectiveness. Microbes can actually feed on protein or polymer additives, so long as they can avoid contacting the isolated silver particles. An easy way to identify a silver protein product is to shake it and watch for foam which lasts for several minutes. Products with these additives are less effective and not real colloidal silver, regardless of a nice color and far higher ppm of silver.
Clear products that contain silver ions with few nanoparticles (known as ionic silver) are the most common ones calling themselves "colloidal silver". Because the particles are so small and have no Tyndall light scattering effect, they are not a colloidal solution. More importantly, unlike nanoparticles, silver ions have a charge and are highly reactive with many substances, causing them not to persist in an effective form. After reacting and becoming bound up in a molecule, the ion can have no effect on a microbe coming in contact. If you spray ionic silver on a surface, it may kill the microbes it initially makes contact with, but soon after another microbe can replace it. Recent papers have reported bacteria that are resistant to silver ions, but no such resistance has been reported about silver nanoparticles.
Real colloidal silver will persist and continue killing microbes. After being sprayed onto a surface, real colloidal silver will keep killing microbes until it is removed. Real colloidal silver won't actually feed the microbes like silver proteins can. Also, the silver nanoparticles in real colloidal silver have nothing coating them to reduce their ability to contact and kill microbes.
The only known discoloration caused by real colloidal silver is from absorption into toe and finger nails which results in a temporary yellow to brown color. This can be avoided by using our 500ppm Silver Cream.
Disclaimer: The statements made here have not been approved by the Food and Drug Administration. These statements are not intended to diagnose, treat or cure or prevent any disease. This notice is required by the Federal Food, Drug and Cosmetic Act.


